Research project
Development and evaluation of strategies to provide longer-term health and social care for stroke survivors and their carers (LoTS-2-Care)
- Start date: October 2013
- End date: September 2018
Description
Objectives and brief methodology
Stroke remains a major illness with at least 900,000 people living in England who have had a stroke. The early stages of the stroke care pathway are becoming more prescribed (treatment in acute and rehabilitation stroke units), but despite policy recommendations, strategies for longer-term care are not developed and stroke survivors and their families face a number of problems and challenges.
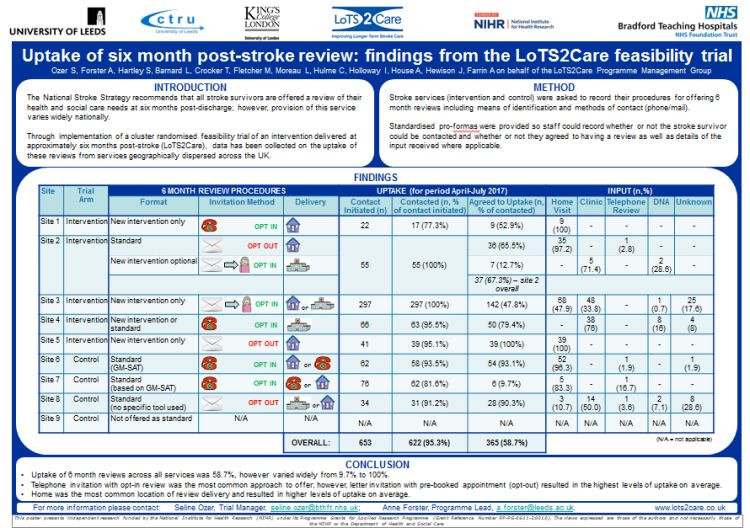
The aim of this programme of work is to develop and evaluate key aspects of a replicable system of longer-term (beyond six months post-stroke) service delivery ‘care strategy’. The emphasis will be on improving quality of life by addressing unmet needs and enhancing participation.
Study 1: This study interviewed stroke survivors and their carers to discuss with them factors influencing unmet needs and things that have helped or hindered their daily lives. Our literature reviews were also updated to identify any effective interventions, and recommended or innovative ideas to support the implementation of the care strategy.
Study 2: A national survey of stroke services was undertaken to gain information on who might provide longer-term care and support.
Study 3: Techniques of intervention mapping were used to develop a care strategy, supporting materials and training programmes (for stroke survivors, carers and staff) using the information obtained from studies 1 and 2.
Study 4: This current work-stream is being undertaken to refine content and test implementation of the care strategy through case studies in three stroke services. Work is also being carried out to assess methods of stroke survivor identification and recruitment to stroke rehabilitation trials.
Study 5: A feasibility cluster randomised trial to refine procedures for a future large scale trial is planned.
This research will address the gap in the stroke care pathway and provide a replicable, and evidence and theory based system of longer-term stroke service delivery.
Participant criteria
All patients with the following characteristics are eligible for the study:
- Have a confirmed primary diagnosis of new stroke
- Are more than three months post-stroke
- Are residing in the community
- Provide written informed consent or Consultee Assent
All caregivers with the following characteristics are eligible:
- Identified by the patient, as the main informal caregiver who provides the patient with support a minimum of once per week.
Publications and outputs
Forster A, Hartley S, Barnard L, Ozer S, Hardicre N, Crocker T, et al. An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomised controlled feasibility trial. Trials (2018) 19(1):317. doi:10.1186/s13063-018-2669-5. PDF https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-018-2669-5
Forster A, Hardicre NK, Crocker TF, Wright A, Burton LJ, Ozer S, Atkinson R, House A, Hewison J, McKevitt C, Farrin AJ and on behalf of the LoTS2Care Programme Management Group. An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for the process evaluation of a cluster randomised controlled feasibility trial. Trials 2018;1119(1):368. PDF https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-018-2683-7
Funding agency
National Institute for Health Research (NIHR)
Further information
For further information contact: Professor Anne Forster on 01274 383406 or by email: a.forster@leeds.ac.uk.
This summary presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Development and evaluation of strategies to provide longer-term health and social care for stroke survivors and their carers, RP-PG-0611-20010). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Link to PDF1: https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-018-2669-5
Links to PDF: 2 https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063-018-2683-7